Featured
- Get link
- X
- Other Apps
Weight Percent To Molarity Calculator
Weight Percent To Molarity Calculator. Admin leave a comment on calculator: The molarity (c m) and percentage (c p) relationship depends on the density of solution (d) along with the molecular weight (m) of the dissolved substance.two equations.
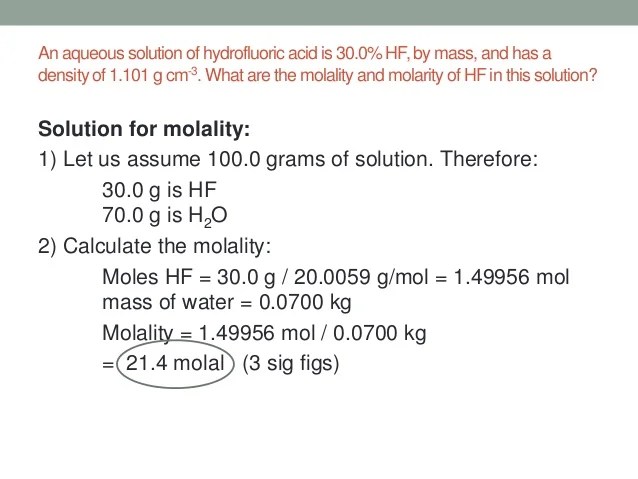
Molarity of an acid or base can be calculated by giving the inputs of molecular weight, density and weight percentage. Percent solutions can take the form of. Substitute the known values to calculate the molarity:
Molar Concentration Is The Amount Of A Solute Present In One Unit Of A Solution.
Molecular mass or molar mass are used in stoichiometry calculations in chemistry. Molarity of an acid or base can be calculated by giving the inputs of molecular weight, density and weight percentage. The two decimal point atomic weights for nickel and chromium are:.
If You Still Don't Sure How To Make Your Life.
Converting molarity to percentage by mass. Weight percent is essentially the same as (i arbitrarily picked grams as the mass unit): Normality(n) = w g / e g × v = equivalent weight per liter / ( w × v ) note that in the above formulae the volume of the solution v should be in in liters.
Its Density Is 1.413 G/Ml And Formula Weight Is 63.01 G/Mol.
Admin leave a comment on calculator: So molarity of the 70% concentrated nitric acid will be. In percent solutions, the amount (weight or volume) of a solute is expressed as a percentage of the total solution weight or volume.
Mass (G) = Concentration (Mol/L) X Volume (L) X Molecular Weight (G/Mol) An Example Of A Molarity Calculation Using.
So 1 g of nacl will be contained in = 100/5 =20 g of solution. % metallic alloy and see what the at. The formula for molarity is simply a conversion of the above expressions.
Concentration Specified On The Left :
% w / w x = g x g s o l u t i o n. Sum the answers obtained under 1. Enter a solution molal (ex:
Comments
Post a Comment