Featured
- Get link
- X
- Other Apps
Using The Solubility Of A Compound To Calculate Ksp
Using The Solubility Of A Compound To Calculate Ksp. The k sp is determined directly from the electrochemical. The first thing to do is identify the values of n and m by writing the dissociation equilibrium for magnesium hydroxide.
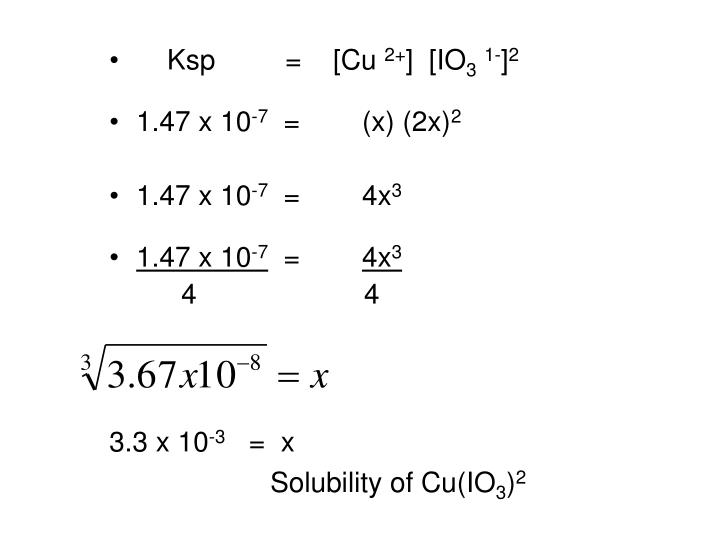
The ksp of a slightly soluble ionic compound may be simply related to its measured solubility provided the dissolution process involves only dissociation and solvation, for example: The solubility (by which we usually mean the molar solubility) of a solid is expressed as the concentration of the dissolved solid in a saturated. First, we need to write out the dissociation equation:
Calculate The K Sp Of Pbf 2.
K s p = [ a. Calculating solubility products from solubilities. For example, at 25ºc, the solubility of pbf 2 is found to be 0.64 g/l.
The Solubility Product Constant, K_(Sp), Represents The Product Of The Molar Concentrations Of Its Dissolved Ions Raised To The Power Of Their Respective Stoichiometric.
6.60 grams of mnf2 will dissolve in. We can use the molar mass to convert from. Ratings 100% (1) 1 out of 1 people found this document helpful;
The Solubility Product Constant Is The Equilibrium Constant For The Dissolution Of A Solid Substance Into An Aqueous Solution.
How to calculate the ksp value Covers the calculations of molar solubility and ksp using molar solubility. The k sp is determined directly from the electrochemical.
The Concentration Of The Fluoride Ion, In Solution At 25°C.
The ksp of a slightly soluble ionic compound may be simply related to its measured solubility provided the dissolution process involves only dissociation and solvation, for example: Solubility product calculator uses solubility product = solubility^2 to calculate the solubility product, the solubility product constant, ksp , is the equilibrium constant for a solid substance. First, we need to write out the dissociation equation:
Calculate How Much Silver Bromide In Moles Will Dissolve In 1 L Of Water At.
Click create assignment to assign this modality to your lms. The first thing to do is identify the values of n and m by writing the dissociation equilibrium for magnesium hydroxide. Relating solubilities to solubility constants.
Comments
Post a Comment